
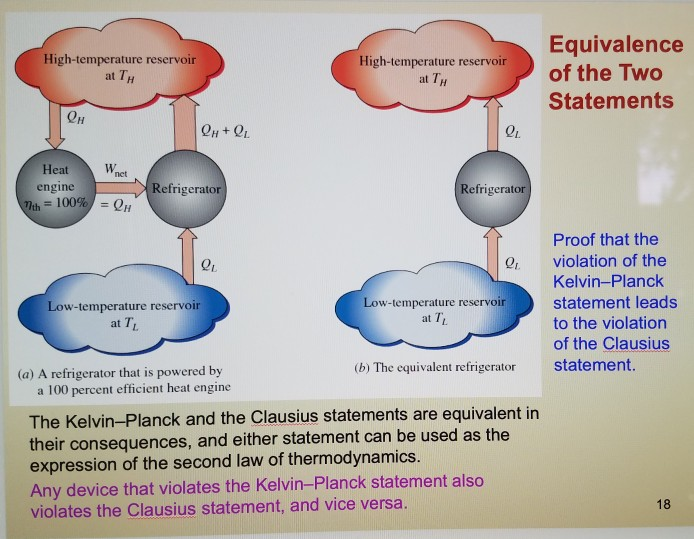
(In contrast, the reverse process, heat transfer from the warmer to the cooler body, is spontaneous and irreversible.) Note that this impossible process does not violate the first law, because energy is conserved. Of course, this process is impossible we never observe heat flow from a cooler to a warmer body. During the process, energy is transferred by means of heat from the cool to the warm body, causing the temperature of the cool body to decrease and that of the warm body to increase. The system is isolated, and consists of a cool body in thermal contact with a warm body. (b) The same, with a device that operates in a cycle.Ĭonsider the process depicted in Fig. (a) Heat transfer from a cool to a warm body. Each asserts that a certain kind of process is impossible, in agreement with common experience.įigure 4.1 Two impossible processes in isolated systems. Both principles are equivalent statements of the second law. What fundamental principle, based on experimental observation, may we take as the starting point to obtain them? Two principles are available, one associated with Clausius and the other with Kelvin and Planck. 4.3.4.Įach of the three parts is an essential component of the second law, but is somewhat abstract. The temperature \(T\bd\) is a thermodynamic temperature, which will be defined in Sec. Third, there is a criterion for spontaneity: \(\dif S\) is greater than \(\dq/T\bd\) during an irreversible change of state. The equation \(\dif S = \dq/T\bd\) allows for the possibility that in an equilibrium state the system has phases of different temperatures separated by internal adiabatic partitions. During a reversible process, the temperature usually has the same value \(T\) throughout the system, in which case we can simply write \(\dif S = \dq/T\). Second, there is an equation for calculating the entropy change of a closed system during a reversible change of state: \(\dif S\) is equal to \(\dq/T\bd\). First, there is the assertion that a property called entropy, \(S\), is an extensive state function. \( \newcommand \)Ī description of the mathematical statement of the second law is given in the box below.


 0 kommentar(er)
0 kommentar(er)
